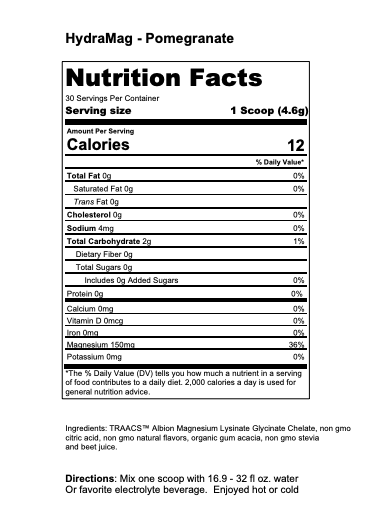
Athlete’s Iron: Iron Bisglycinate Chelate Study Summaries
Iron Bisglycinate Chelate Study Summaries
What can you expect when you add Athlete’s Iron to your training protocol:
Better Performance & Recovery
Iron is critical to supporting blood & muscle oxygenation. Enhancing you body with Iron Bisglycinate Chelate will help your body create a robust oxygenating machine. The more oxygen can be delivered to and in your muscles, the faster you will perform and recover.
First thing first, the form of Iron is essential to absorption and the reduction of adverse events. Adverse events include gut issues; constipation and nausea.
The following studies provide insight to the following benefits:
- Effective Absorption
- Fast Elevation of Reduced Iron Biomarkers: ferritin & hemoglobin
- Reduced Gut Issues
1106-Effectiveness-FeAAC_Pineda-etal1994
This study included 100 adolescents (ages 10-19) suffering from low hemoglobin (<12 g/dl). The subjects were divided into 4 different treatment groups, receiving the iron supplement daily for 4 weeks.:
Group 1: 120mg of iron as ferrous sulfate
Group 2: 120mg of iron as iron amino acid chelate
Group 3: 60mg of iron as iron amino acids chelate
Group 4: 30mg of iron as iron amino acid chelate
After 4 weeks, as subjects had approximately the same improvement in hemoglobin, averaging 2.6g/dl. The subjects receiving 120 and 60mg of iron/day had significant increases in ferritin, with the group on iron amino acid chelate group 120mg iron/day, exhibiting a 25% greater improvement that the ferrous sulfate group.
The group receiving the 60mg of iron as amino acid chelate having a slightly larger increase in ferritin than the ferrous sulfate group. The researchers determined that the iron amino acid chelate was 4 times as bioavailable as the ferrous sulfate. In addition, 33.3% of the ferrous sulfate group having gastric complaints.
The 120mg amino acid chelate group had less than half as many gastric complaints the 60mg amino acid group has less than 1/3 as many gastric complaints as the ferrous sulfate group (and still the same overall effectiveness.
There were zero gastric complaints in the 30mg amino acid chelate group.
1725-Iron_Bioavailability-Pineda TEMA 1999
This article was published in the proceedings from the TEMA conference. The TEMA book was actually published in 2000. The article summarizes a double-blind study on 40 iron deficient infants (ages 3 months to 3 years). Each child received a daily dose (5mg/kg body weight) of iron from either iron amino acid chelate or ferrous sulfate, plus 250 mcg of folic acid, for 28 days. The iron amino acid chelates increased hemoglobin and serum ferritin at a higher rate than ferrous sulfate.
The increase in serum ferritin in the iron amino acid chelate group was significantly higher. This suggests greater bioavailability for iron amino acid chelate, and that it is superior for treating iron deficiency. A calculation for the percentage of iron absorption determined that the bioavailability of iron from the amino acid chelate was 75%, compared to 27.8% for the ferrous sulfate. In addition, the absorption of iron form the amino acid chelate was shown to be regulated by body iron store.
1795-Micronutrient fortified beverage Tanzania Makola
This study included 259 pregnant women (gestational ages 8-34 weeks) in a double blind controlled 8-week supplementation trial. The study was to test the effect of an 11-micronutrient fortified beverage (containing Ferrochel as the iron source) on the hemoglobin, iron, and vitamin status of pregnant women in Tanzania. After 8 weeks on this supplement, there were clinically significant increases in hemoglobin, ferritin, and vitamin status. Researchers concluded that the use of this micronutrient fortified beverage may be a useful and convenient preventive measure to improve the nutritional status of women before and during pregnancy to help avoid potential maternal and fetal consequences of micronutrient deficiency.
1820-Iron and calcium bioavailability Fairweather
This is a review article dealing with the problems associated with trying to fortify foods with iron and calcium. The need for iron forms that are bioavailable and not causing adverse organoleptic changes in the food is discussed. A brief mention of iron bisglycinate chelate is given. Discussing briefly about this iron form being stable in food matrices, and not likely to cause adverse organoleptic changes. In fortifying food with calcium, the problem is not as much about the bioavailability of the calcium used, but the ability to get large enough amounts of calcium into a staple a staple food to make a difference. A very brief mention of calcium citrate malate is given, regarding a study showing its positive impact on slowing bone loss in the elderly, when given with vitamin D.
1576-Comparison-Marchetti2000
This concerns a study that compared the degradation of a mixes of vitamins with either metal sulfates or metal amino acid chelates (from Albion). The mixes were stored at either 20⁰ C or 37⁰C for, and the vitamin potencies were tested at 0, 90, and 180 days. It was determined that there was less degradation of the vitamins prone to oxidation when they were mixed with metal amino acid chelates, and that stored foods with these vitamins and minerals would be more stable when the minerals were mineral amino acid chelates.
1794-Role of iron and factors affecting off color Mellican
The role of highly bioavailable iron in the off-color development of foods and beverages is not well understood. The study set out to determine the interaction of iron with simple phenols and polyphenolics. The researchers developed a food model to investigate this, and then tested their findings on foods containing these substances. The key finding was that using ferrous forms (like Ferrochel) and maintaining them in their reduced form can be achieved by lowering pH, removing oxygen, and the inclusion of reducing agents – one can fortify foods and beverages (containing phenols and polyphenolics) with the highly bioavailable ferrous forms of iron without causing off color development.
1577-Iron-absorption-maize-BovellBenjamin2000
This is actually an article on several different studies concerning iron absorption from ferrous bisglycinate chelate, ferrous sulfate, and ferric trisglycinate from whole maize porridge, as well as from water. The conclusions from the studies are that ferrous bisglycinate chelate is a more effective (higher absorption rate) than ferrous sulfate and ferric trisglcyinate in high phytate foods - like whole maize porridge. Ferric triglycinate was well absorbed when administered in water. The researchers determined that ferrous bisglycinate’s absorption is regulated by the body’s iron status, however, they felt the data indicated that ferrous bisglycinate chelate has a different underlying absorption mechanism than ferrous sulfate.
1607-Sensory-Quality_BovellBenjamin1999
This study demonstrated that maize porridge fortified with ferrous bisglycinate chelate scored the best of the 4 iron forms evaluated in both expert descriptive sensory evaluation and lipid peroxidation (rancidity) level as demonstrated by hexanal production (hexanal is a major decomposition product of the polyunsaturated fatty acids found in maize porridge). The other 3 iron forms included were ferrous sulfate, iron trisglycinate, and NaFeEDTA (an unfortified control was included in the study). The study looked at 2 levels of fortification and stored the products at 3 different temperatures over 20 days. This study further found that adding BHA to a ferrous bisglycinate chelate fortified maize porridge decreased the hexanal production after 20 days to levels below those seen in the unfortified control. The researcher’s previous success with the effectiveness of ferrous bisglycinate chelate in fortification of maize product led to this further look at ferrous bisglycinate chelate. Its high bioavailability in maize product makes it a good possibility for fortification of these kinds of high phytate foods.
1117-Bioavailability-iron-glycine_Fox1998
In this published letter to the editor from American Journal of Clinical Nutrition, Dr. D. Ashmead criticizes the conclusions reached in a published study by TE Fox, et al. Dr. Ashmead states that the study did not actually measure bioavailability and did not demonstrate that iron glycine chelate is dissociated in the gut and therefore handled the same as ferrous sulfate. He states that since all the subjects in the study were iron sufficient, a true difference in absorption could not have been measured. The study showed that both iron glycine chelate and ferrous sulfate have their absorption regulated by body iron stores. Reference is made to a study by Olivares which showed that iron glycine chelate had an absorption 2-2.5 times higher than ferrous sulfate. Dr. Fox responds to Dr. Ashmead’s letter in defense of his conclusions.
In the published study referred to in the letter to the editor, the researchers looked at the bioavailability of ferrous sulfate and iron glycine in various infant foods. The iron forms in this multi-study, were radio –labeled with a stable isotope. The first arm of the study compared the absorption of iron from ferrous sulfate versus iron glycine, when given in a vegetable weaning food. The iron fortified meals were given for 8 consecutive days, and the radio enriched hemoglobin was measure after 14 days. There was no significant difference in the bioavailability of the 2 iron forms. In the second arm of this multi-study, the researchers compare the absorption of ferrous sulfate and iron glycine, using radio-labeling, from 2 different foods. They administered the iron in a low phytate vegetable weaning food, and a high phytate food (whole grain cereal). One group had their vegetable weaning food and multi grain cereal fortified with ferrous sulfate, and the 2nd group with iron glycine. Each group alternated their food forms each day for 8 days. The researchers found no significant differences in the bioavailability of the 2 iron forms in either of the food form. However, the absorption of iron from iron glycine was less affected by the high phytate cereal.
1891-Iron-fortification-UK_FairweatherTait 1997
In this article, the writer discusses the continuous problem of iron deficiency in the UK, despite fortification of flour and some other foods staples. The incidence of iron deficiency is highest in women of child bearing age, children, and adolescents. The writer lists and comments on the relative bioavailability of the known possible iron fortificants. Iron glycine chelate is listed, with a lower relative bioavailability than ferrous sulfate, based on a study by Fox T.E. The comment on iron glycine chelate stated that it is still under evaluation.
1784-Anemia carencial-Queiroz1996
The authors explain the importance of Iron due its important metabolic functions, the normal distribution in the body and homeostasis, based on the regulation of its absorption. A description is given for the types of iron molecules found in food (heme iron and non heme). Factors that act as absorption enhancers, like ascorbic acid, as well as inhibitors like phenolic compounds, fiber and other compounds are reviewed. The iron content in human milk is similar to its content in cow milk, but its absorption is not (human 49% and cow 10%). The recommended ingestion of supplemented iron is 1 mg/Fe/day for the baby from the introduction of the cow's milk, until 24 months of age. Fomon considers that from 1 to 2 years, the child should absorb 0.8 mg/Fe/day. With an absorption of iron stated as 10%, they will need 8 mg/Fe/day, which is almost impossible to get from diet without the introduction of fortified food. In São Paulo city researchers found deficient diet in 48% of children below 5 years. The researchers have shown that the prevalence of iron deficiency anemia in São Paulo was 22% in 1974, and increased 50 to 59.1% in 1991 and 57% in 1994. In 1992/1993 the same group started a study with powder milk fortified with 9 mg/Fe/ 100g in 335 anemic children. Before the intervention 70.7 % of the children were anemic (less than 11 g/Hb/dL) and after the intervention the prevalence of anemia was significantly decreased (to 20.6% and 18%) in the Health Units and in the Day Care respectively.
In 1993 the group started a study based on a new technology that allows fortifying liquid milk. 269 children received 3 mg of iron as iron amino acid chelate for 12 months. Before the intervention the prevalence of iron deficiency anemia was 62.3%. After 6 months this number was reduced to 41.8% and after one year to 26.4%. One of the problems found in the study was the sharing of the fortified milk among other members of the family. This information come from the findings that in the families with just one child the prevalence of anemia dropped from 62.7% to 19.4 %. With 2 children with less 5 years old, the reduction was from 59.8% to 35.6% and in the families with 3 or more children from 72.2 to 33.3%. The researchers concluded that the food fortification with iron is an effective means to minimize the problem of iron deficient anemia.
1488-Use-petit-suisse-Fisberg_translation1995
The study was conducted to looked into an effective way to address the high incidence of iron deficiency in Brazil. In the study, a snack cheese, known as Petit Suisse, was fortified with 2mg of iron, as amino acid chelated Iron (Albion) per serving. The study included 81 children, ages 2-6, who were attending a municipal day care center for children of low socioeconomic class in Sao Paulo. The children received a serving of the Petit Suisse daily for 3 months. Measurements of hemoglobin, hematocrit, and ferritin were done on the children at the start of the study, and after 3 months. The overall iron status of the children improved significantly in the time of the study. The Petit Suisse was well accepted (taste) and tolerated by the children – no signs of gastric intolerance were found. The researchers concluded that Petit Suisse, enriched with amino acid chelated iron may be an excellent choice for the prevention of iron deficiency in pre-school children.
100-Preliminary-fe zn Fairweather 1992
This article summarizes 2 separate rate studies. One study compared ferrous glycinate chelate to ferrous sulfate. The results of these preliminary studies demonstrated that the bioavailability of the ferrous glycinate chelate was significantly greater than that of ferrous sulfate.
1468-Ferric Glycinate Iron bioavailability
The study was intended to compare the bioavailability of ferric glycinate to ferrous sulfate, administered to rats in a basal diet. The bioavailability used hemoglobin replenishment data to compare the relative absorption rates of the 2 iron forms.
The researchers determined that the absorption rate of ferric glycinate was 91% of that seen from ferrous sulfate.
They also felt that ferric glycinate, due to low pro-oxidative tendencies, should be considered for further studies in milk based in formulations – ferrous sulfate had been seen to have bad organoleptics in these formulations.
CFAS52 add 11 Ferrous glycinate chelate specs
This is the monograph of the specifications prepared at the 61st JECFA (2003) and published in FNP 52 ADDF 11(2003) on Albion’s Ferrochel®, which they list as ferrous glycinate (Processed with Citric Acid). Admitting Ferrochel® into the Codex Alimentarius.
JECFA is the Joint FAO/WHO Expert Committee for Food Additives (an international expert scientific committee administered jointly by the Food and Agricultural Organization of the United Nations and the World Health Organization